Schworer, an assistant professor of rheumatology, allergy, and immunology and the UNC School of Medicine, wants to know how lung tissue becomes asthmatic, in the hope of preventing the disease from developing. Breathing trouble can quickly lead to coughing, wheezing, and chest tightness.
UNC collaboration repurposes lab chemical as potential new brain tumor drug
Nobel Laureate Aziz Sancar has teamed up with the NC TraCS-supported SLiCE project to study a potential new treatment for glioblastoma.
Aziz Sancar, MD, PhD
Hearing the word "glioblastoma" can be life-altering for patients and their loved ones. These aggressive brain tumors grow quickly, and can spread throughout the brain, making them extraordinarily challenging to remove with surgery. Because they're in the brain, many medications cannot reach these tumors effectively—and fewer than 10% of adult patients diagnosed with glioblastoma survive more than five years.
Recently, researchers in the lab of UNC molecular biologist and Nobel laureate Aziz Sancar discovered that a common lab chemical called EdU may have potential as a glioblastoma treatment. EdU can cross into the brain and appears to specifically target cancerous cells, potentially helping to shrink these elusive tumors.
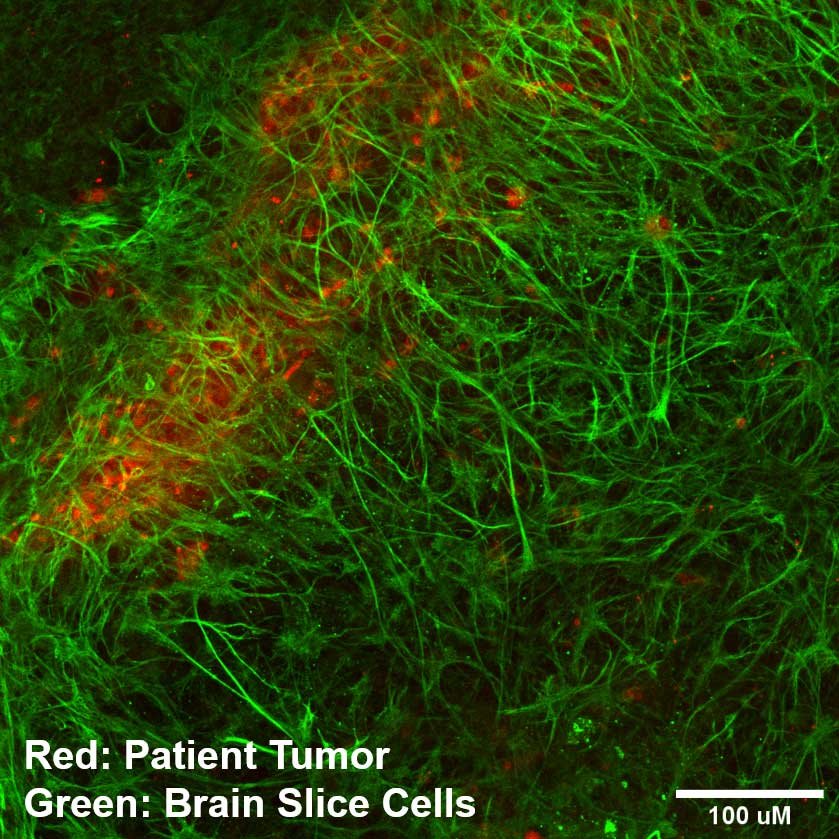
In a recent study, Sancar's lab worked with the Screening Live Cancer Explants (SLiCE) team, led by Andrew Satterlee, a cancer researcher at the UNC Eshelman School of Pharmacy and a current K12 scholar at the North Carolina Translational and Clinical Sciences (NC TraCS) Institute. The SLiCE platform seeds cancer cells or patient tumors on top of living substrates made of brain tissue. This setup maintains tumors in an environment they recognize, helping them act like they do in the patient's body.
Andrew Satterlee, PhD
This technology enabled researchers to get an in-depth look at how EdU affects both healthy and cancerous brain tissue—and take a meaningful step toward the possibility of developing a new medicine for a debilitating disease.
Treating brain cancer is especially complicated because of the blood-brain barrier, a layer of cells that prevents many substances from reaching the brain. This layer helps to keep things like pathogens out of the brain—but it can also keep out many medications.
Temozolomide is one of the few chemotherapy drugs that can cross this barrier, and when a patient is diagnosed with a glioblastoma, temozolomide is often part of their treatment plan. But more than half of all glioblastoma patients don't respond to the medication, and even those who do don't generally live very long with the disease.
EdU, or 5-Ethynyl-2′-deoxyuridine, was not designed as an anti-cancer drug. Instead, EdU is mostly used as an analogue for thymidine—one of the four building blocks of DNA—so dividing cells recognize it as thymidine and incorporate it into their DNA. This allows researchers to track things like cell division and DNA repair by following where EdU is taken up.
Sancar and his colleagues have used EdU to study DNA repair, and a few years ago, they discovered that when EdU is incorporated into a cell's DNA, the cell sees it as a damaged section of genetic code and tries to repair it. The research team speculated that if a cell had a regular supply of EdU and consistently kept adding more EdU to its DNA instead of thymidine, that could trigger a vicious cycle of endless DNA repair, eventually causing the cell to die.
That's where the idea of using EdU as a cancer treatment was born. EdU is incorporated into a cell's DNA when that cell divides and replicates—and cancerous cells divide and replicate far more often than normal cells. The researchers hypothesized that if a person was dosed with EdU, cancerous cells would take up EdU more often than normal cells, essentially targeting the cancerous cells for the vicious cycle of DNA repair and eventual death. What's more, EdU can cross the blood-brain barrier, meaning it could potentially be used to reach tumors that sit deep inside the brain.
Humeyra Kaanoglu and Adebimpe Adefolaju
A recent paper tested this hypothesis with a series of experiments, led by Humeyra Kaanoglu, a PhD candidate in Sancar's lab, and Adebimpe Adefolaju, the translational lead at SLiCE and a PhD candidate in the lab of UNC cancer researcher Shawn Hingtgen. In one experiment, the team dosed various cancer cell lines with either EdU or temozolomide to compare how well each substance killed those cells. In all the cell lines tested, the EdU was more lethal than temozolomide.
In another experiment, the team tested how well each substance worked at treating two different models of glioblastoma in mice. In mice with the first type of tumor, known as CT2A, animals treated with EdU survived significantly longer than animals treated with regular thymidine or animals who went untreated. In mice with the second type of tumor, known as U251, both low and high doses of EdU extended average survival compared with thymidine, although the last mouse in each treatment group ultimately died around the same time.
Humeyra Kaanoglu
The final part of the study used SLiCE technology. SLiCE engrafts living patient tumor tissue, taken right from operating rooms at UNC, atop substrates of living brain tissue in the laboratory. This helps the patient's cancer cells live outside the body much like they would inside a living, functioning brain. This gives researchers an easier method of studying brain cancer than using whole animal models, where the brain is tucked away inside a living creature—and much more insight than research done on an artificial layer of cells grown in a dish that has no architectural similarity to a real brain.
Sancar says he heard about the SLiCE lab while his team was working on some of the initial stages of this new research. "I learned that I have my colleagues across the street who are working on the brain slice," he says. "It is a great system, and I contacted Andrew and asked if we could collaborate, and he was very open to the idea."
"We've been honored to work on this project with Dr. Sancar and his amazing team, and we are excited about the work we're continuing to do with them," Satterlee says.
"I knew the science would be the most meaningful if it could be connected to patients... a platform to test EdU directly on living patient tissues felt like an extraordinary opportunity."
"When we set out to test the therapeutic potential of EdU in glioblastoma, I knew the science would be the most meaningful if it could be connected to patients," Kaanoglu says. "Discovering that Shawn Hingtgen and Andrew Satterlee had a platform to test EdU directly on living patient tissues felt like an extraordinary opportunity."
The team used SLiCE as a platform for growing cancer cells and actual segments of brain tumors removed from patients at UNC hospitals. This setup showed that EdU could pass through the healthy brain cells to reach cancerous cells growing on top—and selectively kill more of the cancerous cells than the healthy cells.
In addition, EdU was effective at killing a portion of many of the tumor segments excised from patients, though not the entire segment. Temozolomide also killed a portion of some of these tumor segments, but EdU had a higher drug sensitivity score (a measure that combines how deadly a drug is to cancer cells and how safe it is for healthy cells) than temozolomide in three of the five tumors tested.
Adebimpe Adefolaju
"For a compound that is otherwise used as a DNA labeling agent, EdU's therapeutic performance on uncultured patient tissue was very exciting," Adefolaju says. "Every time I analyzed data, I was like a bright-eyed deer full of anticipation."
The team is now conducting some follow-up studies to explore additional facets of EdU's potential as an anti-cancer drug. While the results so far are encouraging, there's still a long way to go before EdU could reach the clinic. Any new drug undergoes a series of rigorous clinical trials to ensure it is both safe and effective in actual patients, not just tumors growing in a lab.
But for glioblastoma, which has long evaded researchers' efforts to find new therapeutics, this study offers a meaningful step forward—and the properties EdU has shown here make it an exciting candidate for potential drug development.
"It is a wonderful feeling to think someday soon, EdU could be an added arsenal in the anti-cancer toolkit," Adefolaju says. "Being part of that initial development is not only surreal but an absolute honor."'
"To see this fruitful collaboration reveal that EdU may one day help people facing such a devastating diagnosis is deeply moving," Kaanoglu adds. "For me, knowing that our work could touch a patient's life and offer even a little more hope is the most fulfilling part of doing research."

Sancar and his team in the lab (Credit: Sam Foley)
NC TraCS is the integrated hub of the NIH Clinical and Translational Science Awards (CTSA) Program at the University of North Carolina at Chapel Hill that combines the research strengths, resources, and opportunities of the UNC-Chapel Hill campus with partner institutions North Carolina State University in Raleigh and North Carolina Agricultural and Technical State University in Greensboro.