Quick translation of discoveries, ideas, and inventions into marketable products. R&D and research commercialization support.
FastTraCS, UNC-Chapel Hill's pioneering MedTech incubator, specializes in medical device and technology development through collaborative engineering.
We bring together experts from across clinical disciplines to develop groundbreaking solutions aimed at improving patient care and enhancing community health outcomes.
If you're passionate about advancing medical technology and have a concept to share, join us in our mission to revolutionize healthcare through innovative engineering and collaboration, aiming to uplift and empower the communities across North Carolina.
Specialties
FemTech
FastTraCS is at the forefront of FemTech—a field dedicated to developing technological solutions that address unique unmet needs in women. Our work in women's health includes extensive expertise in Obstetrics & Gynecology and other specialties. Our actively growing portfolio aims to introduce breakthrough solutions that address critical needs. Our strong network of healthcare provider innovators and collaborators uniquely positions us to affect a meaningful change on the future of women's health.
Interventional Technologies
We pioneer development of Interventional Technologies designed to enhance precision and efficacy across medical procedures. Our work includes surgical tools, minimally invasive devices, and diagnostic technologies that enable clinicians to deliver targeted treatments more effectively—improving patient outcomes, reducing healthcare costs, and minimizing length-of-stay.
Translational AI
Translational AI at FastTraCS is aimed at bridging the gap between computational research and clinical utility. We harness the power of machine learning algorithms to analyze complex medical data, enhance diagnostic accuracy, and tailor treatments to individual patient needs. By integrating safe, robust AI into healthcare, we strive to provide physicians with the tools to provide faster, cheaper, and better medical services.
Design & development partners
FastTraCS leverages collaborations with leading institutions and CDMOs to enhance our research and development capabilities.
Our key partnerships include NC State University's Center for Additive Manufacturing and Logistics (CAMAL), and industry leaders such as Protolabs, Nocturnal Product Development, Blur Product Development, and Boldtype—each bringing specialized expertise that complements our in-house capabilities and accelerates the commercialization of new medical technologies.
What we deliver
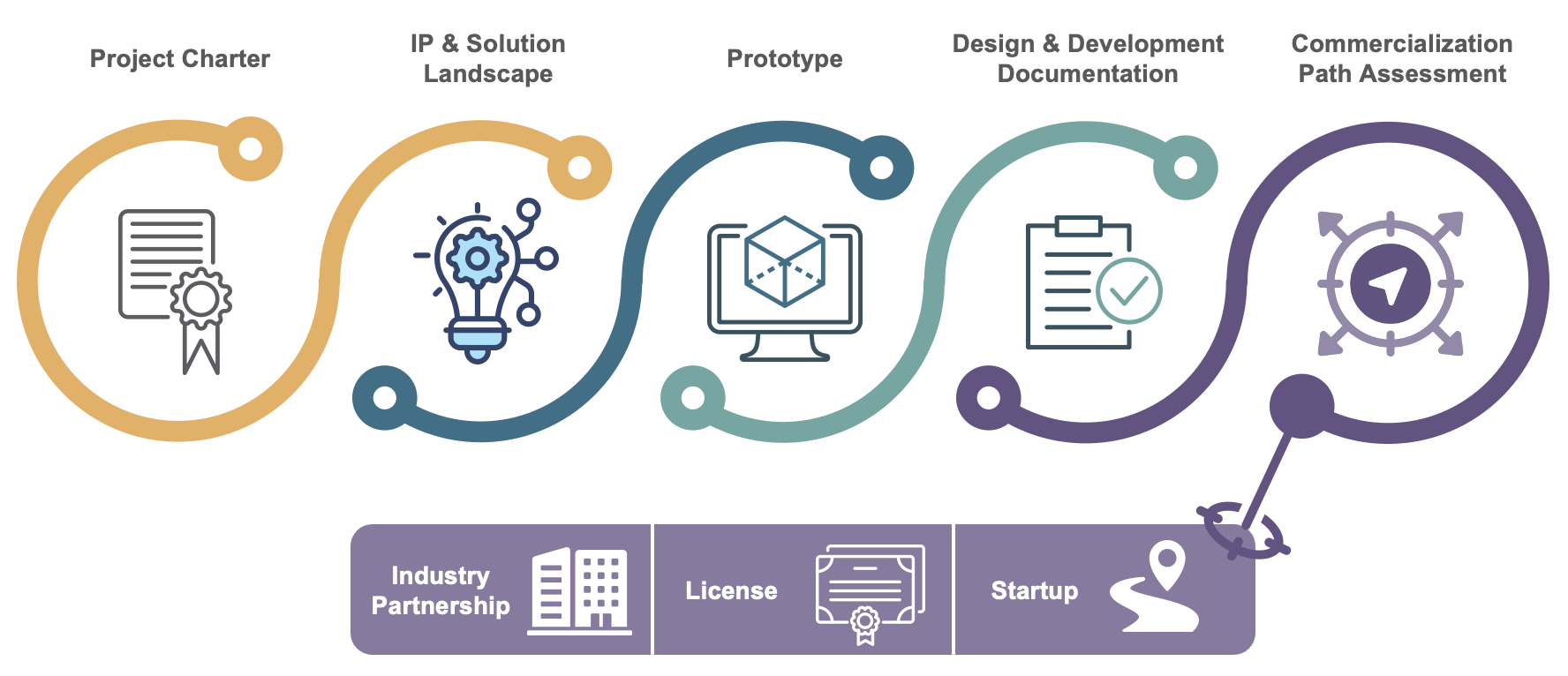
Fig. What we deliver: Project Charter » IP & Solution Landscape » Prototype » Design & Development Documentation » Commercialization Path Assessment [Industry Partnership / License / Startup]
About us
Founded in 2017 with support from NC TraCS, FastTraCS stands as UNC-Chapel Hill's pioneering MedTech incubator, implementing a novel approach to innovation in healthcare technology. Developed originally by Shawn Gomez, EngScD, the program uses a Needs-Driven Innovation framework to align real-world demands of patients and healthcare providers with tailored solutions. This approach, adapted from Stanford's Biodesign program, ensures that technologies are strategically designed from the onset to provide tangible benefits during the early stages of design.
FastTraCS continues to evolve and grow our team. With over 67 years of combined expertise in medical device development, medicine, and commercialization, our team is committed to continuously sourcing new ideas and forming dynamic, passionate teams. Together, we develop groundbreaking medical solutions that tackle critical needs, enhancing patient wellness throughout North Carolina and beyond.
For requesters with a UNC ONYEN or TraCS Connect account:
If you do not have a UNC ONYEN or TraCS Connect account, request an account first, and then return after approved to submit a request.
related services
more information

BLOG: Shawn Gomez
Exploring ideas, systems, and innovations transforming biomedicine and healthcare
Read
BLOG: Andy Kant
Exploring ideas, systems, and innovations transforming biomedicine and healthcare
Read



