Funds the development of innovative solutions to scientific and operational roadblocks in translational research.
Clinical & Translational Science (CTS) Research Program
The NC TraCS Clinical & Translational Science (CTS) Research Program fuels groundbreaking translational science research to fast-track the discovery and implementation of more treatments, for all people, more quickly.
CTS Research Program
As part of UNC Chapel Hill's Clinical and Translational Science Award (CTSA)—funded by the National Institutes for Health (NIH) and implemented by NC TraCS—the CTS Research Program supports cutting-edge translational science research that addresses challenges along the translational research pipeline.
Our goal is to support the development of innovative solutions to scientific and operational roadblocks in translational research that speed up the discovery and implementation of effective treatments. We aim to achieve this by funding investigators and their teams to implement translational science research projects through our Innovation to Impact Awards. These projects:
- Enhance translational research effectiveness and efficiency with hypothesis-driven CTS
- Integrate CTS with clinical and translational research
- Address critical CTS questions with potential for rapid implementation at UNC Health and beyond
Innovation to Impact Awards
Request for Applications
The CTS Research Program invites applications for our CTS Innovation to Impact Awards. Proposals must apply a translational science aim to a translational research question—in other words, proposals will focus on developing innovative methods or approaches that enhance the translational research process while also addressing a specific disease or condition.
Key information:
- $125,000-$250,000 per year in direct costs over a period of 2-3 years (max 3 years)
- Collaboration with NC TraCS resources and services is strongly encouraged
- Awards operate as Cooperative Agreements, with NC TraCS providing substantial involvement and oversight
- Full Proposals were due February 27, 2025. Stay tuned for future cycles and the next RFA release
Questions? Contact the CTS Research Program staff at This email address is being protected from spambots. You need JavaScript enabled to view it..
Although translational research (TR) bridges the gap between basic science and health outcomes for specific diseases or targets, translational science (TS) develops general scientific or operational approaches applicable across various diseases or targets.
Applications to the CTS Innovation to Impact Awards should include a TS aim applied to a TR question. This means projects should focus on developing innovative methods or approaches that enhance the research process while also addressing a specific disease or condition.
Translational research vs. translational science:
Translational Research (TR)The endeavor to traverse a particular step of the translational process for a particular target or disease |
|
Translational Science (TS)The field of investigation focused on understanding the scientific and operational principles underlying each step of the translational process |
|
Source: grants.nih.gov/grants/guide/pa-files/PAR-21-293.html
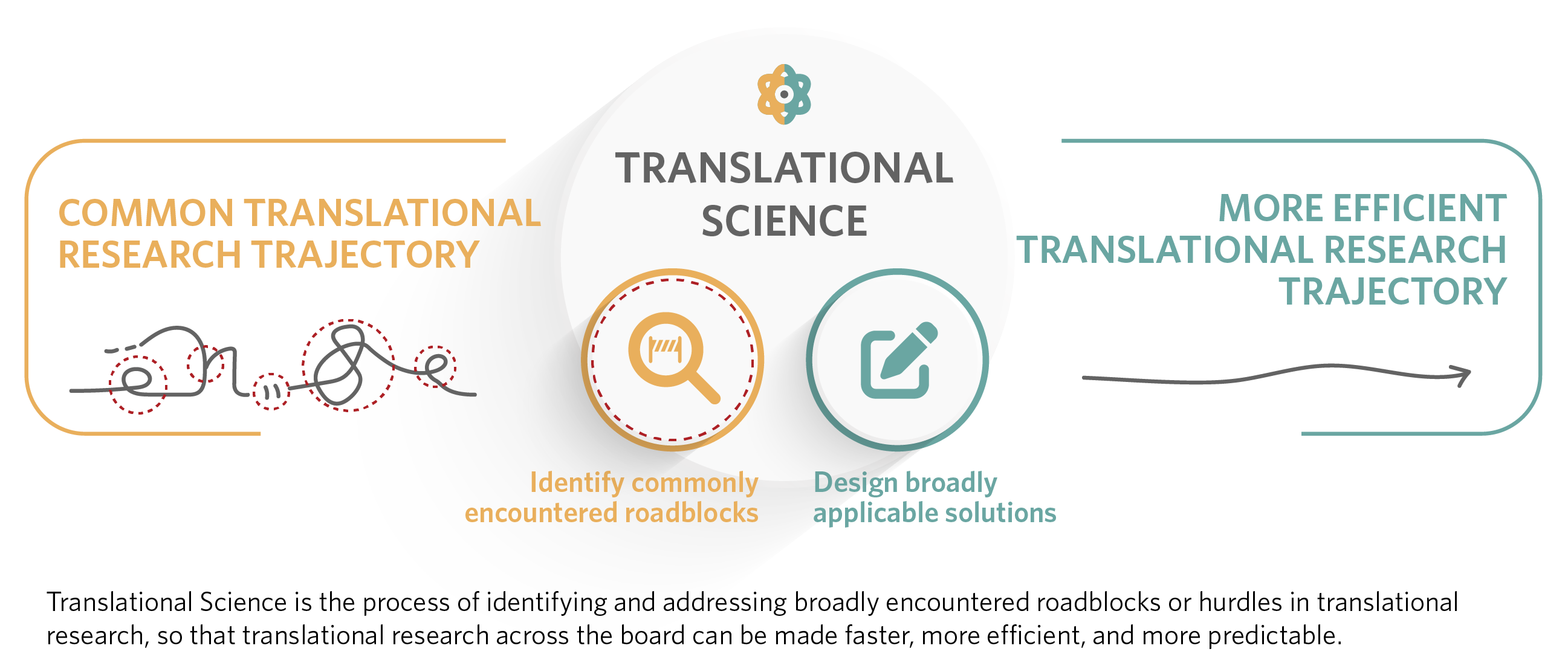
Applications submitted to the TraCS CTS Pilot Program must focus on translational science ; translational research projects are generally not allowed. However, the proposed research may use a specific use case to test a CTS hypothesis (see above), in which case the CTS relevance of the work should be clearly described.
The CTS Research Program that started in 2023 currently supports:
Wayfinding for Prognostic Modeling
Using factors from medical records along with clinician judgement, statistical analyses, model verification, and risk-targeted care plan development in a wayfinding process, this team is developing an oncology (TR use-case) risk-stratified intervention system (OR-SIS) (TS). They aim to demonstrate that OR-SIS is acceptable to clinicians and patients, feasible to implement, and reduces Acute Care Events (ACEs) and prolonged therapy use. It will serve as a prototype for prognostic modeling in other clinical contexts (TS).
This project will result in:
- A generalizable process that will serve as a translational science prototype for prognostic modeling in other clinical contexts (TS)
- Validation of this new process (TS)
- Demonstration of its acceptability and feasibility for implementation (TS)
This project team collaborates with TraCS' IDSci and Biostatistics programs.
Other examples of TS include:
- Development of new research methodologies and/or new technologies/tools/resources that will increase the efficiency and effectiveness of translation
- Early-stage development of new therapies/technologies with generalizable application to an identified translational roadblock
- Dissemination of effective tools, methods, processes, and training paradigms
- Development and testing of a new method for drug discovery that will accelerate discovery of new drugs in several different disease areas
- Development of a more effective and efficient method for getting evidence into practice that can be applied to several different types of practices and evidence
Projects funded by the CTS Research Program must aim to address a truly significant roadblock in CTS science. A list of common roadblocks:
TraCS CTS Pilot Roadblocks Table (pdf)
CTS Research Program awards may range from $125,000-$250,000 per year in direct costs for an award period of 2-3 years (3 year maximum). No-cost extensions are not permitted, so it is critical that the proposed work can be completed within the funding periods.
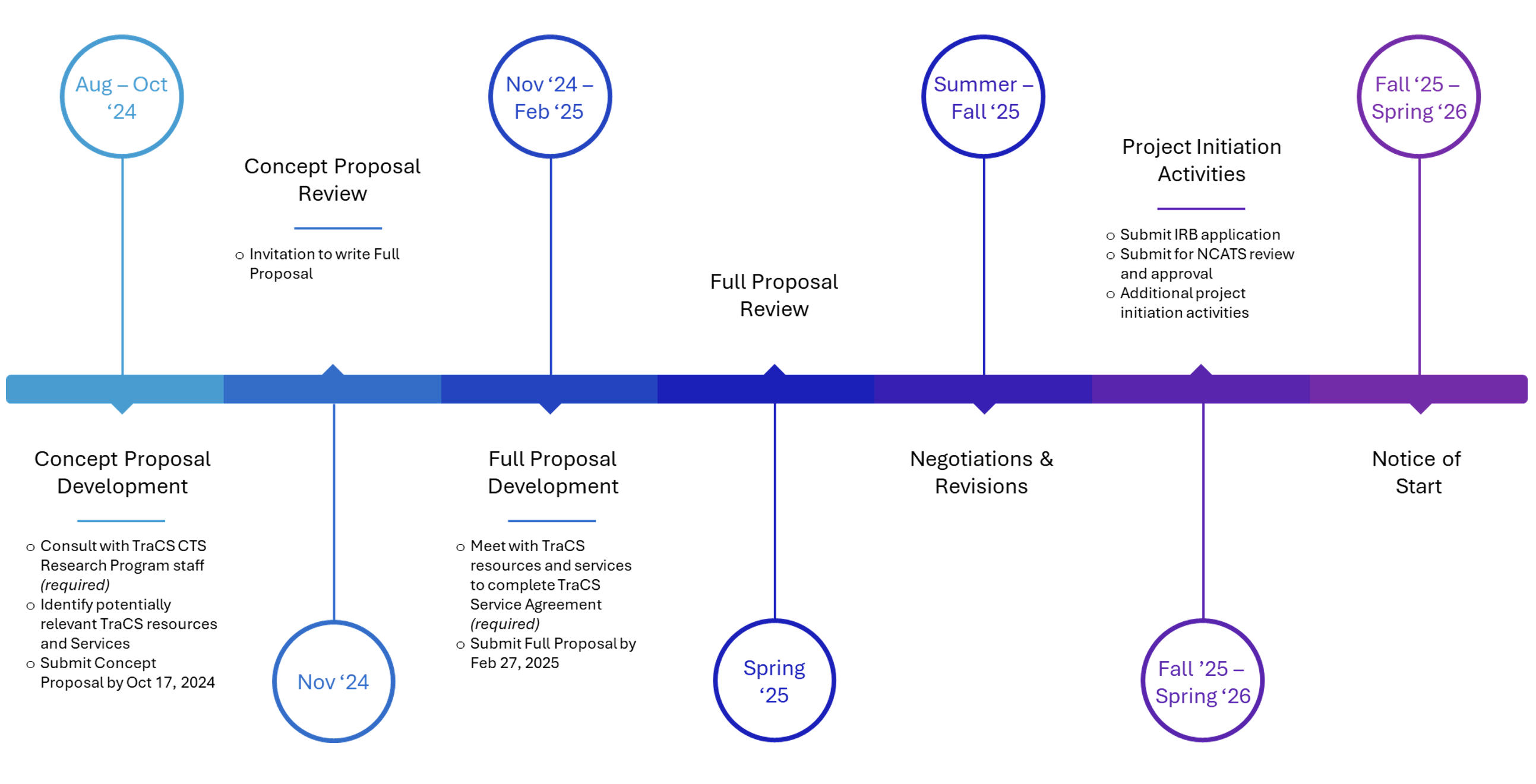
| FOA Release Date | August 2024 |
| FAQ Session | September 13, 2024 |
| Concept Proposal Due Date | October 17, 2024 |
| Invitations for Full Proposals | November 2024 |
| Full Proposal Due Date | February 27, 2025 |
| Review of Full Proposals | Spring 2025 |
| Anticipated Negotiations and Revisions | Summer-Fall 2025 |
| Anticipated Project Initiation Activities | Fall 2025 – Spring 2026 |
| Anticipated Funding/Project Start | Fall 2025 – Spring 2026 |
Concept Proposals are due October 17, 2024, and, if invited, Full Proposals are due February 27, 2025.
The CTS Research Program anticipates to onboard one project beginning in Fall 2025, and a second project beginning in Spring 2026.
To apply as a Principal Investigator (PI) at UNC Chapel Hill, you must have a faculty position, a non-faculty role such as "research scientist," or another independent research position typically eligible for investigator-initiated awards and NIH "R" funding. Post-doctoral research associates are not eligible to apply as PI.
Teams of multiple PIs are encouraged. All PIs must share equal responsibility for the conduct and direction of the project and meet the PI eligibility criteria described above. One PI will serve as the "Contact PI"—the main point of contact with the CTS Research Program administration.
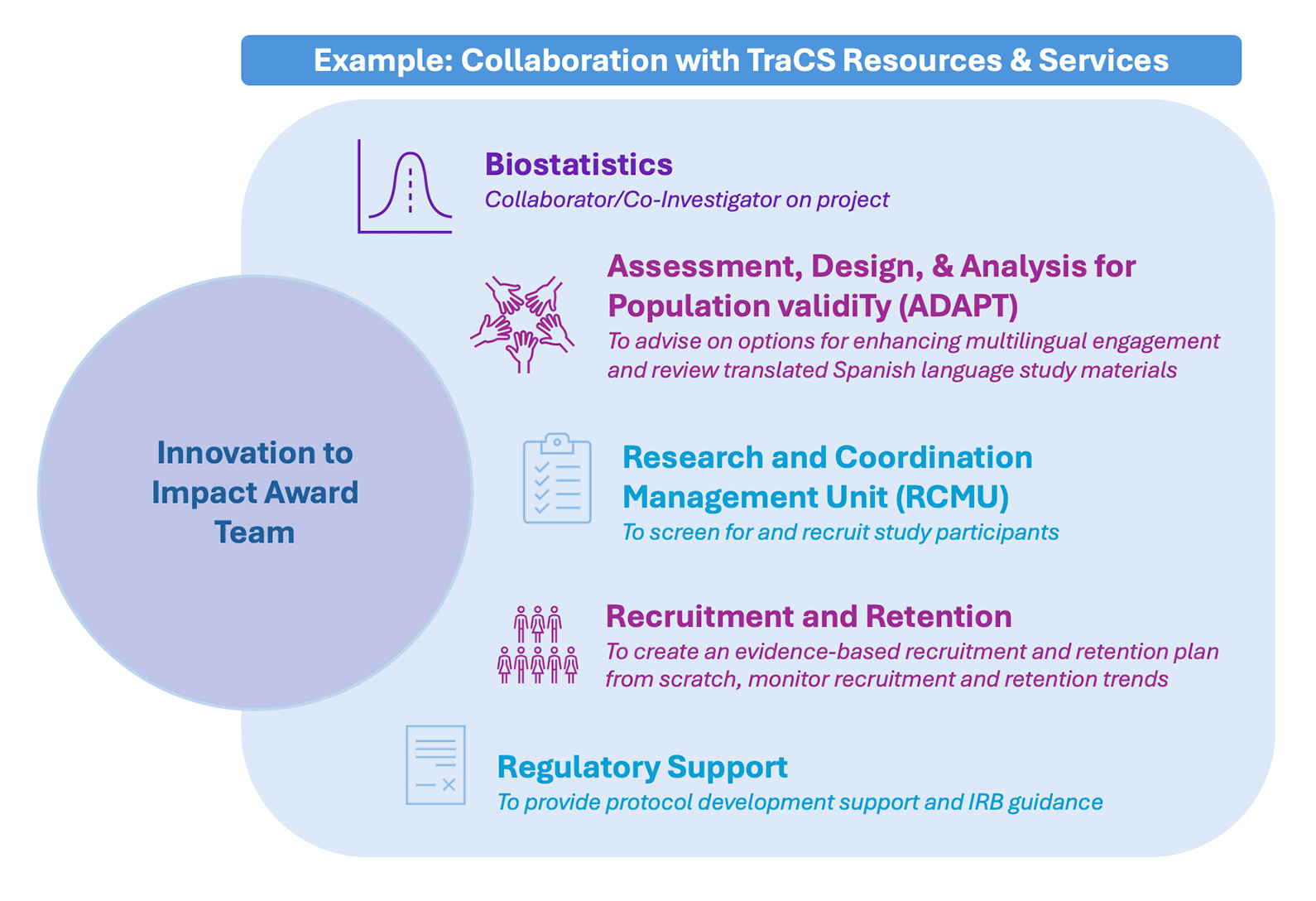
Applicants are strongly encouraged to use NC TraCS resources and services in their proposals. These resources and services will be available to applicants at a reduced rate.
During the Concept Proposal stage, applicants should review NC TraCS resources and services, identify which services they think may be relevant to their project, and briefly describe the role(s) they anticipate the service(s) playing in the proposed work.
Applicants invited to submit a Full Proposal are required to meet with representatives of each of the services they plan to engage with prior to submission. This consult is mandatory, and applicants will be expected to provide detailed descriptions of agreed-upon NC TraCS service assistance.
The applicant must ensure the proposal is clear and logical, convincingly demonstrates the significance of the work, and provides detailed methods for an adequate evaluation.
The following review criteria will be considered during review of the proposal:
- CTS Significance of the work, the translational roadblock it addresses, and its likelihood to advance CTS methods and processes
- Relevance of the CTR use-case to the identified broader translational roadblock
- Novelty/Innovation
- Multidisciplinary team in place that is integral to the conduct of the research
- Soundness of the proposed methods
- Feasibility of accomplishing the stated project goals within the award period
- Plan for implementation of the discoveries in systems at UNC and beyond
- Utilization/Collaboration with NC TraCS resources and services
- Level of community engagement (if applicable)
View the information session video recorded on September 13, 2024.
All Concept Proposals and Full Proposals will be submitted through the NC TraCS online submission system, located under "Clinical and Translational Science Innovation to Impact Awards".
FAQs:
- What is meant by the award being a cooperative agreement?
These awards will function as cooperative agreements, where TraCS will provide ongoing input into the conduct and direction of the work. This will involve the convening of a TraCS support team, comprising a TraCS CTS Research Program Project Manager, faculty content experts if needed, and TraCS resources and services representatives, who will meet regularly with the research team to evaluate progress, identify roadblocks and discuss workarounds, and advise on next steps. NC TraCS expects the project PI to report over the lifetime of the work the outcomes achieved due to the award (e.g., subsequent external funding, publications, presentations and patents). - What about international partners or research?
Funds cannot be used to support research outside of the US. However, data previously generated through international research can be used in projects, as long as data analysis, etc., is conducted domestically. - Can the Innovation to Impact Award fund a clinical trial?
The NIH will not allow the Innovation to Impact award to fund Phase III or later clinical trials. It may fund Phase I and II. Please refer to this decision aid to determine if your study is a Phase III clinical trial. - Do I have to include TraCS resources and services in my budget?
All applicants are strongly encouraged to collaborate with TraCS resources and services. The cost of these services should be included in the grant budget. Remember that the nature and extent of assistance to be provided must be discussed, agreed upon, and documented in the TraCS Service Agreement Form with the appropriate TraCS Service rep(s) prior to Full Proposal submission. CTS Research Program staff can assist applicants in navigating this process, if needed. - Can CTS Research award funds be used to pay consultants?
Yes, as long as the necessity of using a consultant and a description of the skills/services they provide are described in the Budget Justification and detailed in a Consultant's Letter. - Can CTS Research award funds be used to support PI or Co-I salary?
Yes, this award can cover PI and co-I salary up to the NIH salary cap. - Are there any limitations to equipment purchases?
Equipment can be budgeted if its necessity for the proposed work is justified in the Budget Justification. Plans to purchase a large piece of equipment or to spend a significant portion of the budget on equipment should be discussed with Program staff prior to submission. - Should I budget for indirect costs?
No indirect costs may be budgeted for UNC. Indirect costs will be paid based on award funds spent at N.C. A&T or NC State, but these are additional to the award and should not be itemized in the Budget. - When should I start preparing my IACUC/IRB paperwork?
We recommend that you start preparing your regulatory paperwork as early as possible – preferably as soon as you enter into the Negotiations and Revisions stage. Because these awards use NIH funds, they require NCATS regulatory approval, which can only be applied for after institutional (IRB/IACUC) approval has been received. This extra step can add up to 4-5 weeks to the regulatory approval process. - What are the mandatory questions for Concept Proposals?
The following mandatory question must be answered in the application system when submitting your Concept Proposal:
Please identify which of the following TraCS resources and services may be relevant to your project and provide a brief (1-2 sentence) description of how you plan to collaborate [if a box is checked, there will be the option to provide the sentence]:- Research Coordination and Management Unit (RCMU)
- Regulatory
- Recruitment & Retention
- Informatics & Data Science (IDSci)
- FastTraCS
- Translational Science App Development
- Biostatistics
- Assessment, Design, & Analysis for Population validiTy (ADAPT)
- Implementation Science Methods Unit
- Education & Workforce Development
- Clinical & Translational Research Center (CTRC)
- Population Health Data (Previously CER)
- Practice-Based Research (NCNC)
- Qualitative Research Service
- Patient and Community Engagement in Research (PACeR)
- Team Science
- What are the Preliminary Project Readiness questions included in the Full Proposal application?
This component of the application assesses key elements of readiness for project start-up. This will help NC TraCS understand what support you may need for project start-up, should your full proposal be selected to move on to the Negotiations and Revisions round. Refer to the Collaborating with TraCS resources and services - Mandatory Questions for Full Proposals (pdf) to preview this list of questions.
Questions regarding the CTS Research Program and the application process? Please contact CTS Research Program staff at This email address is being protected from spambots. You need JavaScript enabled to view it..
For requesters with a UNC ONYEN or TraCS Connect account:
If you do not have a UNC ONYEN or TraCS Connect account, request an account first, and then return after approved to submit a request.
