How NCATS tackles persistent problems in translation
From the NCATS Director's Corner (June 27, 2023)
 NCATS addresses long-standing bottlenecks in the translational research pipeline so that new health solutions reach people faster.
NCATS addresses long-standing bottlenecks in the translational research pipeline so that new health solutions reach people faster.
NCATS has its sights on long-standing barriers that have slowed progress on health solutions, and we are tackling them head on. In this new Director's Message series, I will share stories that show you the translational science approaches we're using to address the pesky problems blocking the translational research pipeline.
Educating the broader community about translational science and its value was a theme that emerged from our recent strategic planning roundtable discussions. Before I dive into how we use translational science to solve important problems, I want to address a few key points.
Translation, translational research, and translational science are related but different. Translation turns observations in the laboratory, clinic, and community into diagnostics, therapeutics, medical procedures, and behavioral changes that improve people's health. Translational research moves a project to the next step in the translational process. Translational science enables these projects to reach their goals faster and more efficiently. At NCATS, we define translational science as the field that generates scientific and operational innovations that overcome the long-standing barriers along the translational research pipeline. With its focus on improving the process, translational science ultimately leads to more treatments for all people more quickly.
A variety of bottlenecks slow down translation. The Drug Discovery, Development, and Deployment Maps (see Fig. B below) show the long and winding path for a promising therapeutic to reach the medicine cabinet. Scientific and operational hurdles hinder progress at each turn. These barriers include research models that fail at predicting clinical outcomes in people, research processes that are slow and laborious, data platforms that aren't interoperable, and regulatory and administrative burdens that delay study start-up. NCATS' remit is to clear the path so translational research projects move more quickly and easily on their way to becoming health solutions. We do this by developing such solutions as human cell-based research models, platform technologies, data integration and analysis tools, and templated legal agreements.
Translational science principles characterize effective approaches for translation. Our staff have identified an initial set of eight principles. The principles stem in part from in-depth case studies of successful NCATS-supported activities. These activities addressed both scientific and operational processes to make the research faster, more efficient, and more impactful. The principles are:

- Focusing on unmet needs
- Producing generalizable solutions
- Emphasizing creativity and innovation
- Leveraging cross-disciplinary team science
- Focusing on efficiency and speed
- Utilizing boundary-crossing partnerships
- Using bold and rigorous approaches
- Prioritizing diversity, equity, inclusion, and accessibility
These principles are intentionally broad and apply to research anywhere along the translational continuum. We see these principles as a work in progress, and we welcome feedback from our community on them.
The case studies I'll share throughout this series will show you how NCATS is using translational science principles to solve persistent translational problems. My hope is they also will clarify translational science, build excitement around NCATS' problem-solving approaches, and spark ideas for overcoming other translational barriers.
Your partner in science and health,
Joni L. Rutter, PhD
Director
National Center for Advancing Translational Sciences
Figure B.
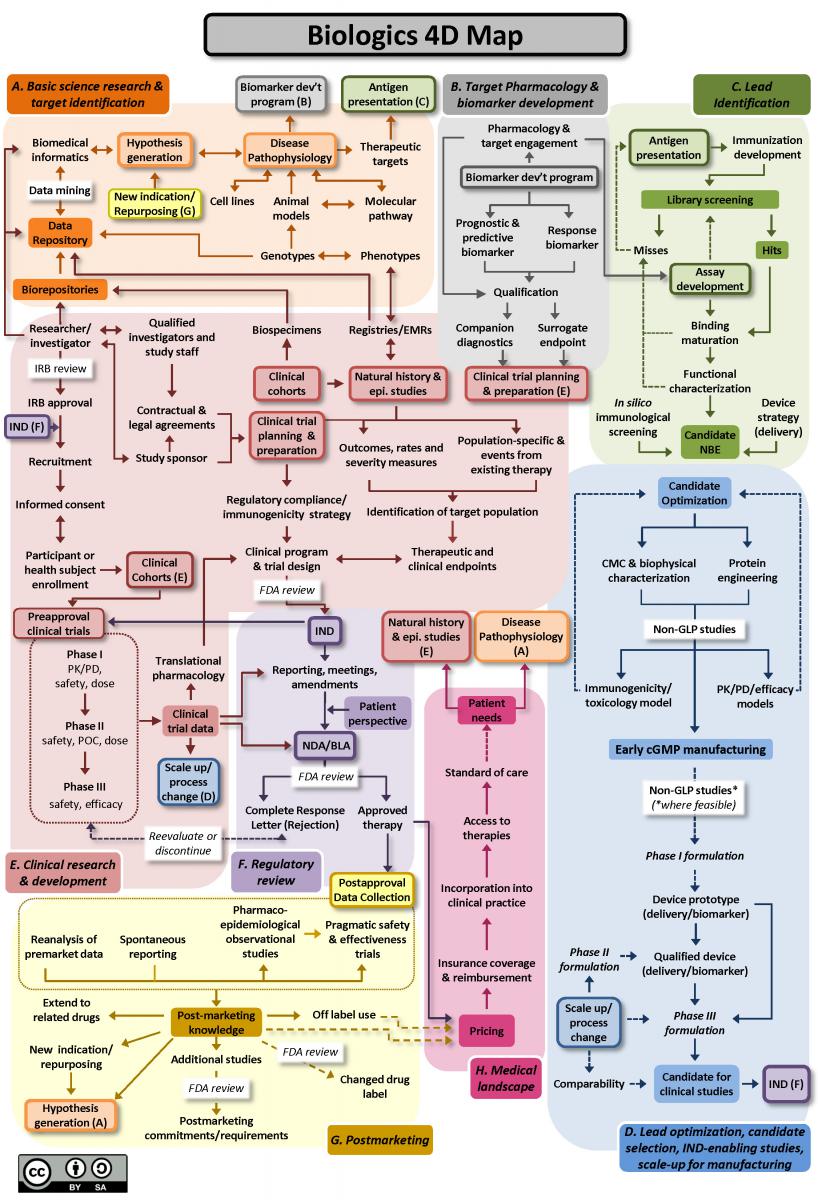
Figure B. 4DM Biologics Map. Map depicting the interconnected nature of key steps in the drug development lifecycle for biologics. The steps are grouped into eight identified neighborhoods, each depicting the steps and processes necessary to advance within a particular stage of development. Steps within each individual neighborhood are frequently dependent upon both other steps within that neighborhood as well as with steps in other neighborhoods, resulting in a complex and nonlinear development process. The figure should be attributed to: Wagner JA, Dahlem AM, Hudson LD, Terry SF, Altman RB, Gilliland CT, DeFeo C, and Austin CP. Drug Discovery, Development and Deployment Map (4DM): Biologics. Available at ncats.nih.gov/translation/maps. Last updated November 2017. Download this map here.
Originally posted at ncats.nih.gov/director/june-2023.
