‘Flying carpet’ technique delivers one-two punch of anticancer drugs
An international team of researchers has developed a drug delivery technique that utilizes graphene strips as “flying carpets” to deliver two anticancer drugs sequentially to cancer cells, with each drug targeting the distinct part of the cell where it will be most effective.
The technique was found to perform better than either drug in isolation when tested in a mouse model targeting a human lung cancer tumor.
The researchers also found that the protein, TRAIL, can bind directly to the surface of cancer cells, which had not been demonstrated previously. The work was done by researchers at North Carolina State University, the UNC School of Medicine, and China Pharmaceutical University.
In this study, the researchers attached two drugs – TRAIL and doxorubicin (Dox) – onto graphene strips. Graphene is a two-dimensional sheet of carbon only one atom thick. Because TRAIL is most effective when delivered to the external membrane of a cancer cell, and Dox is most effective when delivered to the nucleus, the researchers wanted to deliver the drugs sequentially, with each drug hitting a cancer cell where they could do the most damage.
The Dox binds to the graphene due to similarities in the molecular structure of the drug and the graphene. The TRAIL is bound to the surface of the graphene by a chain of amino acids called peptides.
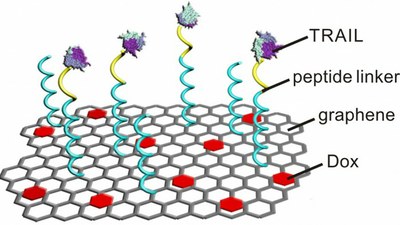
“These drug-rich graphene strips are introduced into the bloodstream in solution, and then travel through the bloodstream like nanoscale flying carpets,” said Zhen Gu, PhD, assistant professor in the joint UNC-NCSU biomedical engineering program at NC State and senior author of a paper published in the journal Advanced Materials.
Once in the bloodstream, these flying carpets take advantage of the fact that cancer tumors cause nearby blood vessels to leak; the grapheme strips use those leaks to penetrate into the tumor.
When the flying carpet comes into contact with a cancer cell, receptors on the cell surface latch onto the TRAIL. Meanwhile, enzymes that are common on the surface of cancer cells sever the peptides linking the TRAIL to the graphene. This allows the cell to absorb the Dox-laden graphene and leaves the TRAIL on the surface, where it begins a process to trigger cell death.
After the flying carpet is “swallowed” by the cell, the acidic environment inside the cell promotes the separation of the Dox from the graphene – freeing it to attack the nucleus.
“We’ve demonstrated that TRAIL itself can be used to attach a drug delivery system to a cancer cell without using intervening material; this is something we didn’t know,” Gu said. “And because graphene has a large surface area, this technique enhances our ability to apply TRAIL to its target on cancer cell membranes.”
The researchers tested the drug delivery technique in preclinical trials against human lung cancer tumors in mice. The technique was significantly more effective than Dox or TRAIL by themselves, or a combination of Dox and TRAIL in which the peptide link between the graphene and the TRAIL couldn’t be severed.
“We’re now trying to secure funding to support additional preclinical studies in order to determine how best to proceed with this new technique,” Gu says.
First author of the paper is Tianyue Jiang, PhD, a former graduate student in Gu’s lab who is now on faculty at Nanjing Tech University. The co-corresponding author is Ran Mo, PhD, a former postdoctoral researcher in Gu’s lab who is now on faculty at China Pharmaceutical University. Co-authors include Wujin Sun, a PhD student in Gu’s lab; Qiuwen Zhu, a PhD student at China Pharmaceutical University; Nancy Burns, a PhD student at NC State; and Saad Khan, PhD, PhD, Alcoa Professor of Chemical and Biomolecular Engineering at NC State.
This research was supported by the North Carolina Translational and Clinical Sciences Institute (NCTraCS) and with funding from NC State and UNC-Chapel Hill.
Originally posted by UNC Health Care on January 12, 2015.