Erin Carey, MD, MSCR, the new FastTraCS co-executive director, will be a bridge between clinicians at UNC and the engineering and commercialization team at the North Carolina Translational and Clinical Sciences (NC TraCS) Institute—with the goal of expanding collaboration and innovation across the university.
New FastTraCS co-executive director wants to help UNC clinicians turn ideas into solutions
FastTraCS is a passionate group of biomedical engineers who support UNC Health physicians, nurses, and staff by identifying needs and problems in clinical settings and creating innovative, technological solutions.
Erin Carey, MD, MSCR, will be a bridge between clinicians at UNC and the engineering and commercialization team at the North Carolina Translational and Clinical Sciences (NC TraCS) Institute—with the goal of expanding collaboration and innovation across the university.
Erin Carey, MD, MSCR
When Erin Carey, MD, MSCR, first joined UNC as a gynecological surgery fellow more than 10 years ago, she found that many of her patients suffered from some form of chronic pain. But she also quickly learned that the field of pain medicine doesn't always have good answers when it comes to gynecologic pain.
"One mentor told me it's the Bermuda Triangle," Carey says.
Carey, now the director of the Minimally Invasive Gynecologic Surgery Division at UNC, has spent a good chunk of her career trying to fill that clinical gap—including working on a project with FastTraCS, the biomedical engineering and commercialization team at NC TraCS, to develop a new physical therapy device for patients with pelvic floor muscle pain. The device, still in development, could one day enable patients to alleviate chronic muscle pain through physical therapy from the comfort of their own home.
This month, Carey is also taking on a new role as co-executive director of FastTraCS, in large part out of a desire to help other medical professionals find, interrogate, and resolve similar gaps in everyday medicine.
"My investment with the FastTraCS team is really to better incorporate FastTraCS across UNC," Carey says—engaging more clinicians and researchers in the process of innovation and design to help turn problems into ideas and tangible solutions.
After a short stint away following her fellowship, Carey returned to UNC in 2016. Shortly after her return she began working with FastTraCS on the new physical therapy device. When a patient has myofascial pelvic pain, a tightness in the muscles on the base of the pelvis, a physical therapist will often use a wand to massage the muscles. This process can ease the pain, but it comes with some drawbacks.
For one, people have to take time off work, find and travel to a specialty therapist's office and pay for an appointment, Carey says, creating a series of potential barriers for some patients. In addition, myofascial pain therapy can be very intimate, so the patients aren't exactly excited to attend these sessions, she adds.
Many clinicians know the feeling of watching their patients face a similar barrier to care, or even struggling with a recurring challenge in their own practice. Maybe they've even thought of a potential solution to the problem—like Carey did with the idea for a pelvic floor release wand—but lack the expertise or resources to make the solution a reality, especially since medical innovation can often require a lot of time and money.
By joining FastTraCS, Carey is hoping to make that process simpler and more efficient for researchers and clinicians. "Sometimes you don't need or want to take 30 years to get somewhere," she says.
The first step in creating a new device is identifying a potential need or problem to be solved, no matter how routine that need might be. "Sometimes, with medicine, we do things because it's the way it's always been done—and it's not always the best or the right solution," Carey says.
Carey first engaged with the FastTraCS team at a design thinking workshop held to help clinicians find specific pain points in their clinical practice, as well as areas for potential improvement and missing links in how they treat patients. In Carey's case, while she came to FastTraCS with the idea for a device, the team started by taking a step back and identifying the underlying clinical need—and she says this can be important for determining the best path forward. For one, maybe a device or solution to the problem already exists, and clinicians only need to learn about it. Similarly, if a similar, but not perfect, device exists, can it be adapted to solve the problem at hand?
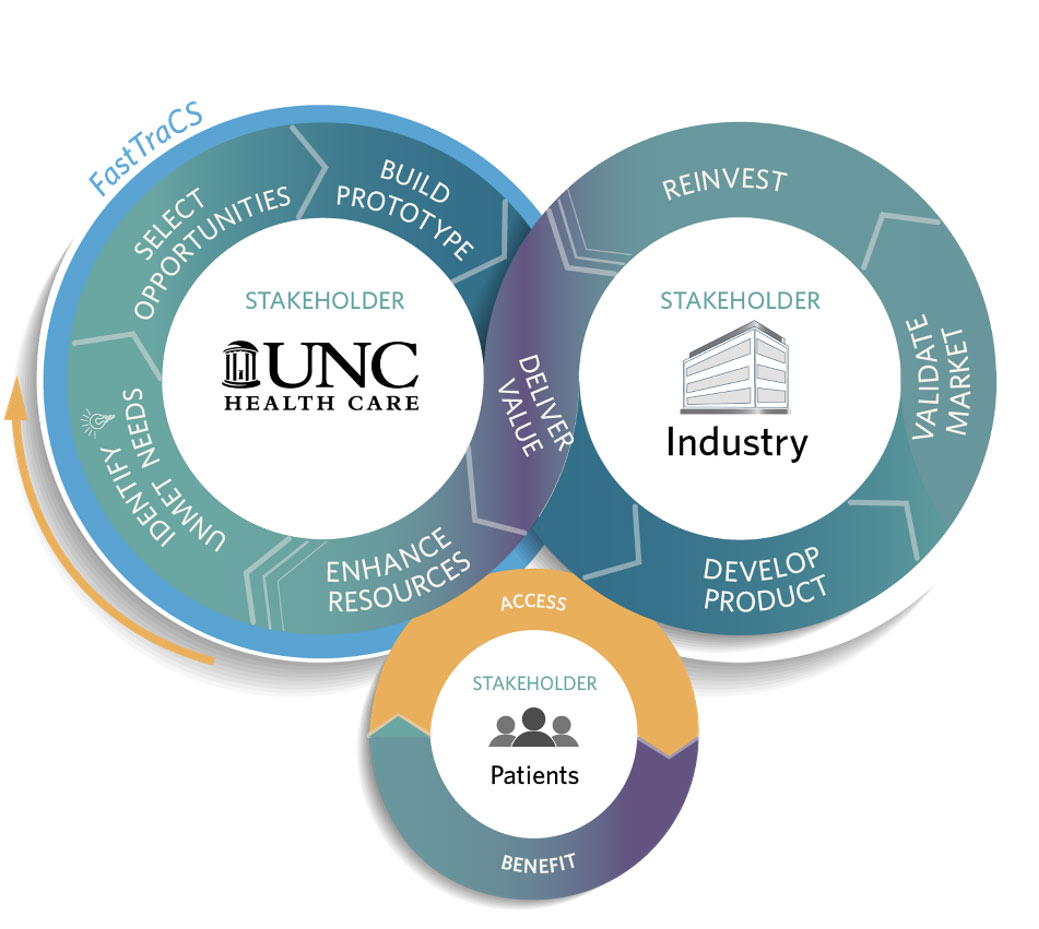
If the need-identification process reveals that there isn't a potential solution out there already, that opens even more questions—like how to commence the research needed to develop a medical device, how to get that device on a path toward FDA approval, and how to secure funding to make that research and approval a reality.
That's where FastTraCS' engineering and commercialization expertise come into play, helping researchers navigate the complicated world of design and regulation. "What we want to do is make it a lot easier, make the pathways a little bit more clear," Carey says.
The goal of a FastTraCS project is to translate an idea into a workable prototype that could be used for a potential clinical study. But Carey wants FastTraCS to become the "go-to" resource for Class 1 and Class 2 medical devices at UNC—and in addition to their in-house expertise, FastTraCS can offer funding to support research and connect clinicians to other experts, like the UNC Office of Technology Commercialization. This network can provide researchers with direction and momentum toward commercial success and regulatory approval even after the FastTraCS process ends.
By working with the FastTraCS team over the past few years, Carey and her colleagues have now created a functioning prototype for the interconnect pelvic floor wand. Therapists can program the device to each patient's specific needs and track their progress via a smartphone app, which guides the patients through completing their own therapy at home and tracks their symptoms.
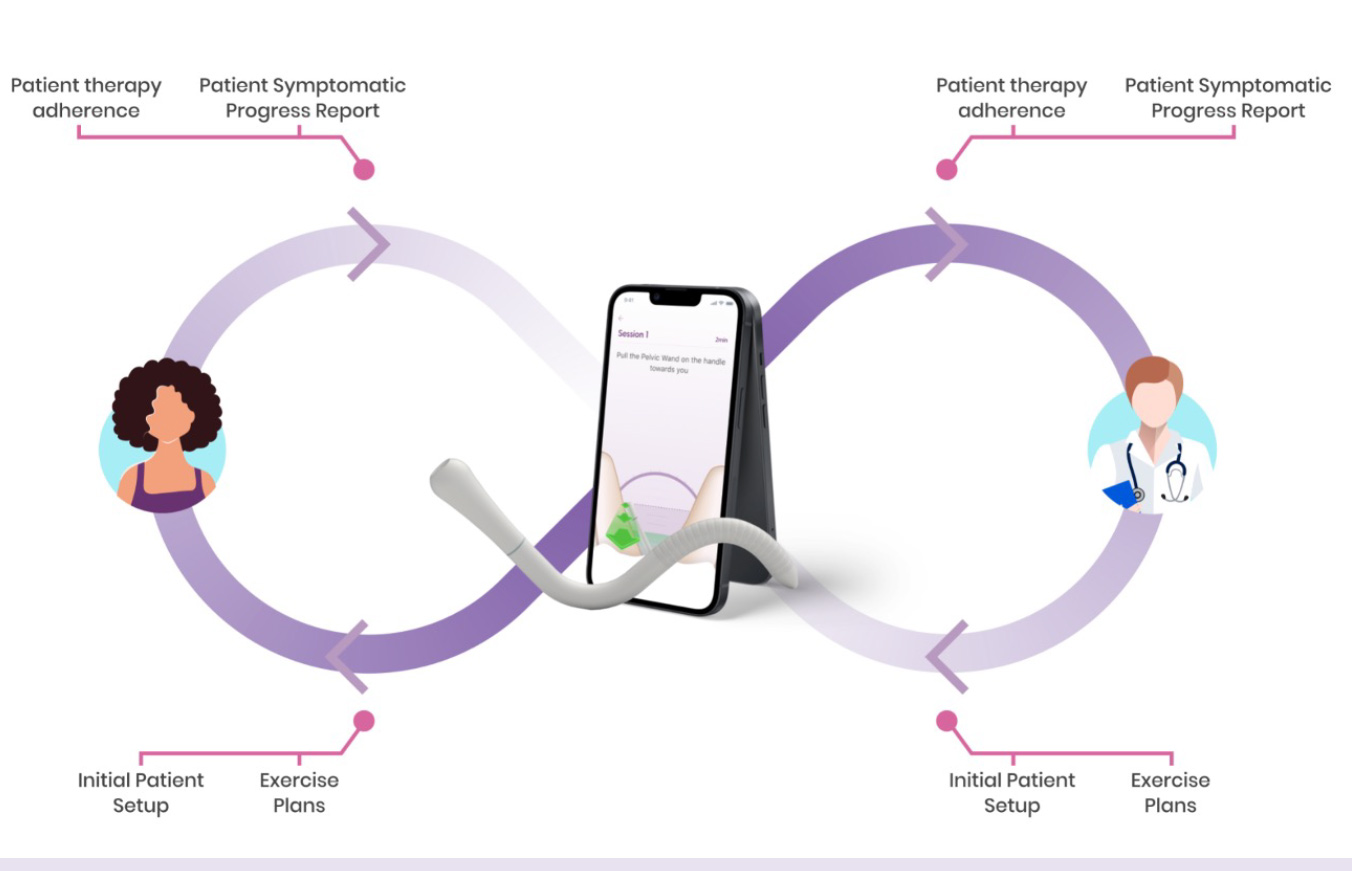
Fig. A mockup of the pelvic floor wand and smartphone app, and how it fits into the process of patient care
In addition, Carey and her colleagues have also incorporated a start-up company, completed an assessment of the existing market for similar devices and applied for a patent. Currently, they're finishing up a Phase 1 Small Business Innovation Research grant on the new wand, with an application on the way for Phase 2.
There's still a long way to go before the product might reach shelves, but Carey is quick to note that none of the success she's had so far would have been possible without the FastTraCS process. And in her new role, success will be about providing other researchers with the resources and pathways to achieve similar wins.
"What success would look like, to me," Carey says, "is not only getting more clinicians engaged in this process and thinking in an innovative, solution-driven way—but actually getting some of these ideas to a commercial product that actually can then get on the shelves and help patients."
More FastTraCS articles
NC TraCS is the integrated hub of the NIH Clinical and Translational Science Awards (CTSA) Program at the University of North Carolina at Chapel Hill that combines the research strengths, resources, and opportunities of the UNC-Chapel Hill campus with partner institutions North Carolina State University in Raleigh and North Carolina Agricultural and Technical State University in Greensboro.